What are PRRs?

PRRs – pattern-recognition receptors
PRRs are proteins that allow the host to recognize microbes. Fundamental to this process is the possibility to differentiate self from non-self-structures. In the case of PRRs conserved structures on the microbes are recognized as foreign. Examples of such structures includes for example nucleic acids, cell wall components and flagella. Collectively these are called microbe-associated molecular patterns, short MAMPs. The direct responsiveness of host cells to MAMPs is generally referred to as innate immunity. This leads to the release of mediators such as cytokines, chemokines and anti-microbial peptides from the affected cell which recruit and activates cells of the adaptive immune system and can destroy microbes directly, respectively. PRRs are expressed by nearly any cell of the human body and allow the host to react in a cell-autonomous manner to pathogens, without the need of cells of the adaptive immune system. However, activation of PRRs is fundamental to adaptive immune responses.
This concept was coined by Charles Janeway in 1986, who recognized that an initial trigger, provided by the innate immune system, is needed to drive adaptive immune responses. This is one of the reasons why adjuvants needs to be given for some vaccinations to enhance immunogenicity.
A prominent example of a MAMP is bacterial lipopolysaccharide (LPS), also known as endotoxin. This substance from the cell wall of Gram negative bacteria is recognized by the PRRs TLR4 and an intracellular receptor. This process is involved in the live threatening cytokine storm seen in patients with sepsis. In 2011 Jules Hoffmann and Bruce Beutler were awarded the Nobel Prize for the characterization of the Toll protein in Drosophila and the Toll-like receptor TLR4 receptor in mammals in innate immune responses.
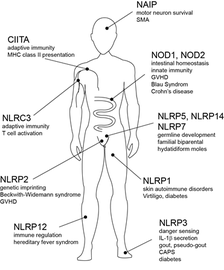
Besides TLRs, that recognize MAMPs in the extracellular lumen, there are PRRs that reside in the cytosol of the cell and react towards invading microbes. These are nucleic acid recognizing proteins and most importantly members of the nucleotide-binding domain and leucine-rich repeat containing (NLR) family of proteins. 22 NLR proteins have been identified in humans, one of the best characterized being the NOD1 and NOD2 proteins that have been well characterized as receptors for bacterial peptidoglycan components. A wealth of recent work has shown that these NLRs play essential roles in the immune defence against several Gram negative and Gram positive pathogens. Furthermore, they have been recognized to contribute to immune and tissue homeostasis, in particular in the gut. The importance of NOD2 is underlined by certain inheritable mutations in the NOD2 gene which are associated with severe inflammatory disorders. NOD2 polymorphisms can give a predisposition to the severe inflammatory bowel disease Crohn’s disease, while other mutations give raise to a rare sever systemic inflammation (Blau-Syndrom). Important physiological roles are also shown for other NLR members (Fig. 1).
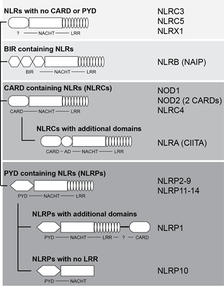
NLR proteins have a typical tripartite domain architecture. In most cases, they possess an N-terminal death-domain fold domain (PYD or CARD), a central typical ATPase domain (NACHT) and C-terminal leucine rich repeats (LRRs) (Fig. 2).
Our current knowledge suggests that interaction of MAMP is mediated by the LRRs which subsequently leads to oligomerization via the NACHT domain which ultimately frees the N-terminal domain to allow for interaction with adaptor molecules to induce signal transduction processes.
Still, the function of many human and murine NLR proteins remains elusive.
The functional characterization of these proteins will help to understand the imitation and regulation of innate immune processes and provide new clues for the development of new therapeutic interventions for the treatment of inflammatory disorders.